Heat removal and catalyst deactivation in a Sabatier reactor for chemical fixation of CO2: Simulation-based analysis
Heat removal and catalyst deactivation in a Sabatier reactor for chemical fixation of CO2: Simulation-based analysis
Abstract
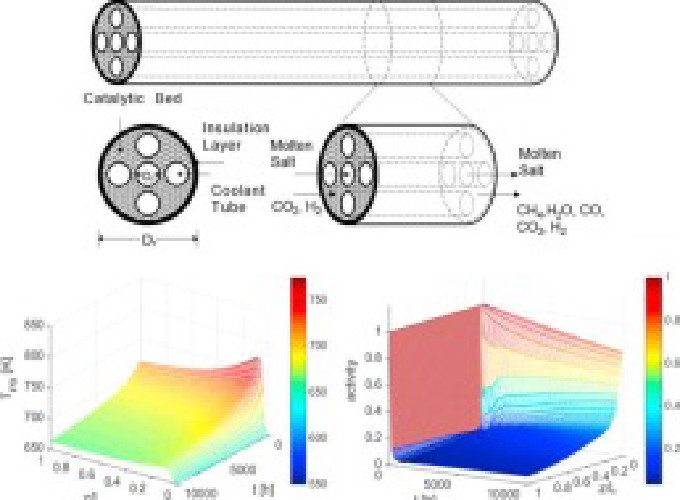
Thermo-catalytic hydrogenation of CO2 into synthetic CH4 via Sabatier reaction is an attractive route to reduce fossil fuels consumption and to limit greenhouse gas emissions, while potentially providing revenue. Hydrogen required for the reaction can be generated by water electrolysis using renewable or low carbon footprint electricity. With respect to CO2 methanation, a number of technological challenges have to be resolved in order to make this technology economically viable. The highly exothermic nature of the Sabatier reaction makes heat removal a challenging task. Another major problem is catalyst deactivation by coking caused by CH4 cracking at elevated temperatures. Maximizing CH4 production over extended periods of operation will require highly efficient heat removal to facilitate CH4 production, while minimizing catalyst deactivation. In this study, a heat-exchanger type, molten salt-cooled packed bed reactor is analyzed using a transient mathematical model. The model considers inter-compartment heat exchange and catalyst deactivation by coking, assuming the use of a Ni/Al2O3 catalyst. The reactor performance was investigated in terms of CO2 conversion and CH4 yield for the case of the feed containing CO2 and H2, and for the case of the reactor fed with biogas (40% CO2 and 60% CH4) and H2. The model predicts that, though the catalyst deactivation leads to a substantial decline in the reactor performance, CH4 yields and CO2 conversions over 80% are achievable after 10,000 h of operation.