Mediating interaction strength between nickel and zirconia using a mixed oxide nanosheets interlayer for methane dry reforming
Mediating interaction strength between nickel and zirconia using a mixed oxide nanosheets interlayer for methane dry reforming
Abstract
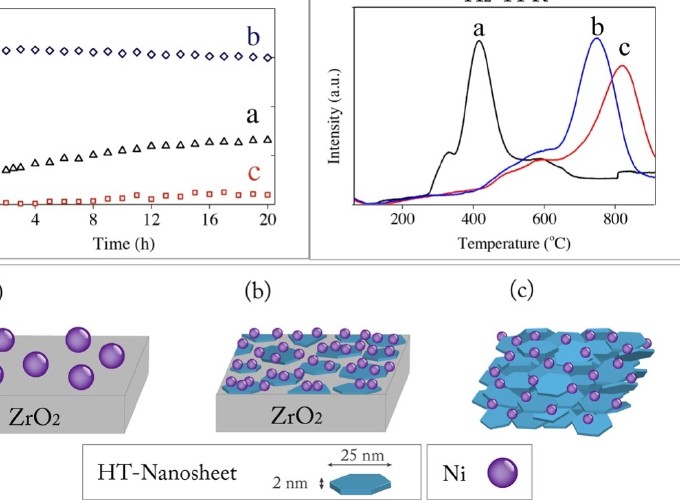
For many reactions balancing the degree of interaction between the metal catalyst and the underlying support material is pivotal for obtaining optimal catalytic performance. One important example is the reforming of methane contained in biogas, using CO2 as an oxidizer, to make syngas (H2 and CO). For this reaction to become industrially viable, the catalyst needs to be based on non-precious transition metals. Unfortunately, catalyst deactivation induced by metal sintering and coking remains a major challenge for this class of catalysts. Herein, we demonstrate the use of a ″surface phase oxide″ in the form of MgAl mixed oxide nanosheets (<2 nm thick), derived from layered double hydroxide, as an interlayer to mediate the interaction between nickel nanoparticles and an underlying zirconia support. This unique hierarchical catalyst configuration is shown to enhance conversion, under undiluted conditions and GHSV=240,000 cm3h−1gcat−1, by up to 2-fold, as compared to Ni supported on ZrO2 and by up to 15-fold as compared to Ni on bulk MgAl mixed oxide, keeping the catalyst stable over 240 h reaction times. To gain better understanding into the mechanism of enhanced catalytic performance we used a combination of PXRD, SAXS, Cryo-TEM, H2-TPR, STEM-EDS, and XPS in conjunction with the results for the catalytic performance. The combined results show that the amplified catalytic performance is strongly correlated with the modification of the metal-support interaction (MSI) by the MgAl mixed oxide interlayer. Although the physical interaction of Ni particles at the edges of the nanosheets with the underlying ZrO2 cannot be completely dismissed, all the available data shows no such interaction exists. Hence, the change in MSI in the stacked configuration is attributed to the indirect MSI effect of the underlying ZrO2 on the properties of the nickel catalyst. Overall the approach described here, for manipulating MSI to promote the challenging methane dry reforming, can be extended by replacing the underlying metal oxide support (commercial or custom made), synthesis of mixed oxide nanosheets of different A2+B3+ ratios and type, and changing the active metal catalyst.