Thermal management of a Sabatier reactor for CO2 conversion into CH4: Simulation-based analysis
Thermal management of a Sabatier reactor for CO2 conversion into CH4: Simulation-based analysis
Abstract
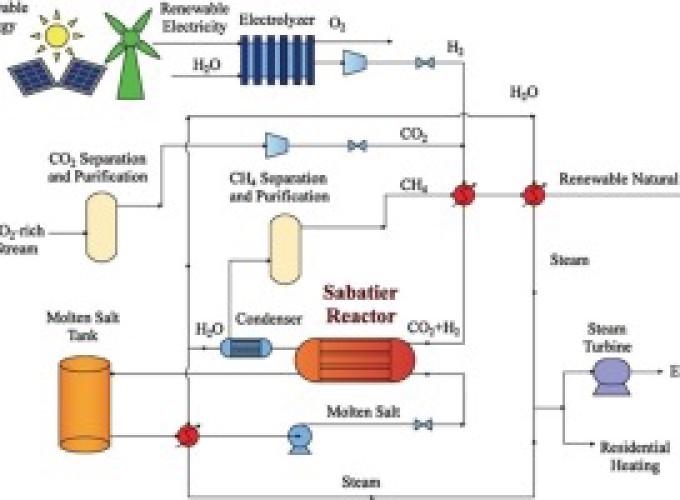
Converting CO2-rich waste streams such as raw biogas, landfill gas and power plant flue gas into synthetic fuels and chemicals will reduce greenhouse gas emissions, providing revenue at the same time. One option is to convert CO2 into CH4 by hydrogenation via Sabatier reaction. This synthetic methane will be renewable if the H2 required for the reaction is generated via water electrolysis using solar and wind energy or hydroelectricity. However, to realize the potential of this approach, a number of technological challenges related to the Sabatier reactor design have to be resolved, including thermal management. The high exothermicity of the Sabatier reaction can lead to reactor overheating. High temperatures are unfavorable to the exothermic and reversible methanation process, resulting in low CO2 conversions. A simulation-based study of a Sabatier reactor was performed in order to optimize the removal of heat, while maximizing CO2 conversion and CH4 production. The heat-exchanger type packed bed reactor with internal cooling by a molten salt was simulated using a transient, pseudo-homogeneous mathematical model. Reactor performance was evaluated in terms of CO2 conversion and CH4 yield. The simulation results show that feed temperature, feed flow rate, and molten salt flow rate are the crucial parameters affecting the reactor performance. For the optimized operating conditions, the model predicts CO2 conversions and CH4 yields above 90% at high reactor throughputs, with space velocities up to 10,000 h−1. A preliminary techno-economic evaluation is provided: opportunities and challenges are outlined.