An effective delivery is a key factor in applying novel molecular therapeutics to disease treatments. Current drug delivery relies on technologies of active targeting and efficient cell penetrating. Among various delivery methods, peptide-mediated drug delivery has emerged as a promising means in anticancer drug delivery technologies. Peptides are versatile and easily designed to incorporate a number of specific attributes, such as cell targeting, membrane penetrating and controlled release. We have been studying self-assembling peptides, EAK16s, for hydrophobic anticancer drug delivery. These peptides have a unique amphiphilic structure with alternating negative and positive charges; they can interact with both hydrophobic and hydrophilic molecules. Such peptides have been found to form stable nanostructures at wide range of temperatures and pHs. They are also biocompatible and biodegradable; no significant immune responses were observed when introduced into animals. Moreover, their self-assembly can be controlled by various physicochemical conditions ( SY Fung et al., 2003 Biophys. J.; Y. Hong et al., 2003 Biomacromolecules; Y. Hong, et al., 2004 J. Adhesion; S. Jun et al, 2004 Biohpys. J.). All these properties make the self-assembling peptides excellent construct materials for drug delivery.
We have been investigating EAK16-II, a typical self-assembling peptide, as a carrier for hydrophobic anticancer drug delivery. It was found that EAK16-II can interact with a hydrophobic model drug pyrene and some anticancer agents, forming complexes in aqueous solution. The complexation was monitored using fluorescence and light scattering techniques. The surface morphology of the complexes was investigated by scanning electron microscopy (SEM). The encapsulated hydrophobic molecules can be further released into a lipophilic environment, i.e., liposome—a cell membrane mimic. The rate of the release can be controlled by adjusting the peptide-to-cargo concentration ratio during their complexation (Keyes-Baig et al., 2004 JACS). Furthermore, the peptide-drug complexes have been found to have antiproliferation ability to cancer cells, and the peptide itself is relatively non-toxic. This work demonstrated a great potential of the self-assembling peptides as delivery vectors for hydrophobic anticancer drugs.
Techniques:
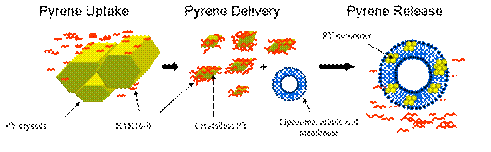
Peptide-mediated delivery of hydrophobic model drug pyrene into liposomes