The surface tension and adsorption kinetics are important in many physical, chemical, mechanical and biological applications, such as foaming, wetting, coating, printing, distillation, extraction, deposition, modification of cell membrane, and proper functioning of the lung surfactants. The presence of a surface-active agent, or surfactant, is crucial to these applications because of its influence on the surface tension. When a fresh interface is formed, the surfactant is adsorbed at the interface to achieve a thermodynamically more stable state. The result is a reduction in the Gibbs free energy of the system and hence a decrease in surface tension.
It is shown that when a volatile surfactant is dissolved in a liquid, it presents a finite partial pressure in the vapor phase, and the surface tension of the solution can be affected by surfactant adsorption from both sides of the vapor/liquid interface. The preliminary results of this research show that at steady-state, the effect of adsorption from the vapor side is more important than that from the liquid side (these results contradict with the behavior of conventional surfactants). The objective of this project is to investigate the effect of surface perturbation and environment conditions (i.e., temperature and pressure) on the surface tension, adsorption kinetics and surface nanoscale structures of a group of short carbon chains alcohols. A modified Gibbs adsorption equation, a modified Langmuir isotherm, a modified kinetic transfer equation and a modified Frumkin equation are derived to model the experimental data of the steady-state and dynamic surface tension. In the new equations the effect of adsorption/desorption of more than one species from both sides of a vapor/liquid interface is considered simultaneously. Equilibrium constants, kinetic rate constants for adsorption from both sides of a vapor/liquid interface and any interaction between different species can be revealed from this modeling.
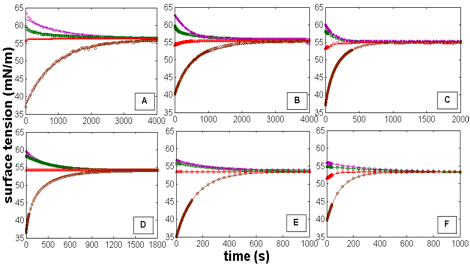
Figure 1. Effect of temperature on the surface tension of 1-hexanol solution [Concentration of the environment solution (Cenv) is 9mM in all experiments, and Concentrations of the drop solutions (Cdrop) are 2mM (![]() ), 5mM (
), 5mM (![]() ), 9mM (
), 9mM (![]() ), and 30mM (
), and 30mM (![]() )]. Each graph represents a different temperature; (A) 10°C, (B) 15°C, (C) 20°C, (D) 25°C, (E) 30°C, (F) 35°C. Solid lines represent theoretical predictions from the modified kinetic transfer equation.
)]. Each graph represents a different temperature; (A) 10°C, (B) 15°C, (C) 20°C, (D) 25°C, (E) 30°C, (F) 35°C. Solid lines represent theoretical predictions from the modified kinetic transfer equation.